Auditing Organization (AO) versus Notified Body (NB) versus Registrar. What's the difference? – Oriel STAT A MATRIX – ELIQUENT Life Sciences Blog
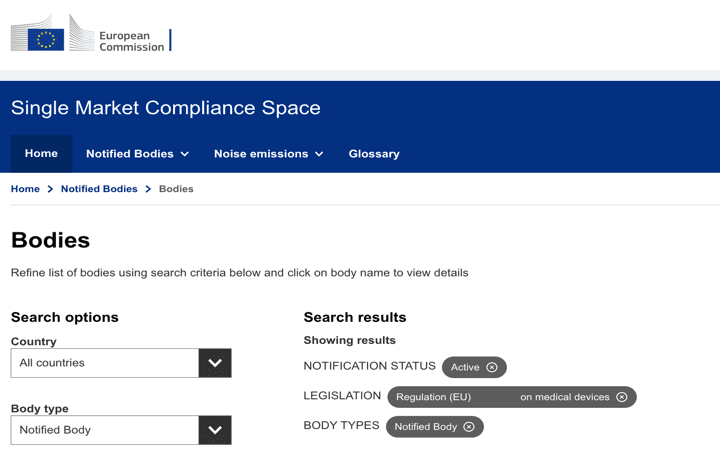
Organismos Notificados MDR (44): RISE – Medical Notified Body AB (Suecia) ON num. 3033 nuevo Organismo Notificado. Enhorabuena !!!

CataloniaBio & HealthTech | Hard Reg Café "Open talk with EU Notified Bodies about the extra transition year to implement MDR" (Regulatory Affairs Workgroup)
![Requirements Relating to Notified Bodies for EU MDR [Video] - LearnGxP: Accredited Online Life Science Training Courses Requirements Relating to Notified Bodies for EU MDR [Video] - LearnGxP: Accredited Online Life Science Training Courses](https://learngxp.com/wp-content/uploads/2021/04/ELM-320-01-Requirements-Relating-to-Notified-Bodies-for-EU-MDR.png)
Requirements Relating to Notified Bodies for EU MDR [Video] - LearnGxP: Accredited Online Life Science Training Courses
![ARTICLE] Why You Need To Review Your Technical Documentation NOW (And 8 Pitfalls to Avoid at all Costs) - Medidee Services ARTICLE] Why You Need To Review Your Technical Documentation NOW (And 8 Pitfalls to Avoid at all Costs) - Medidee Services](https://medidee.com/wp-content/uploads/2022/08/Technical-Documentation-Infographic.png)





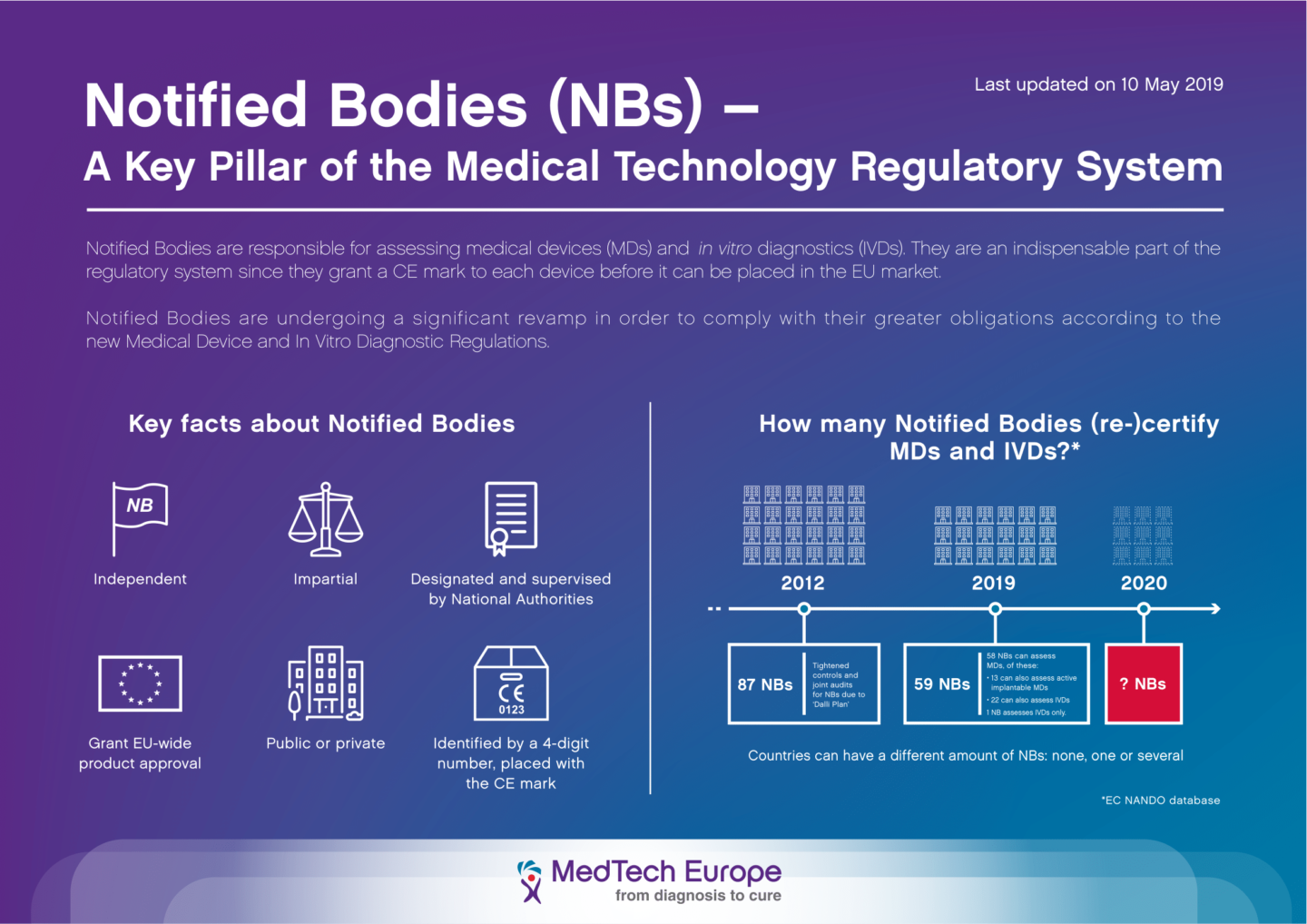
%20On%20EU%20Notified%20Bodies.jpg)