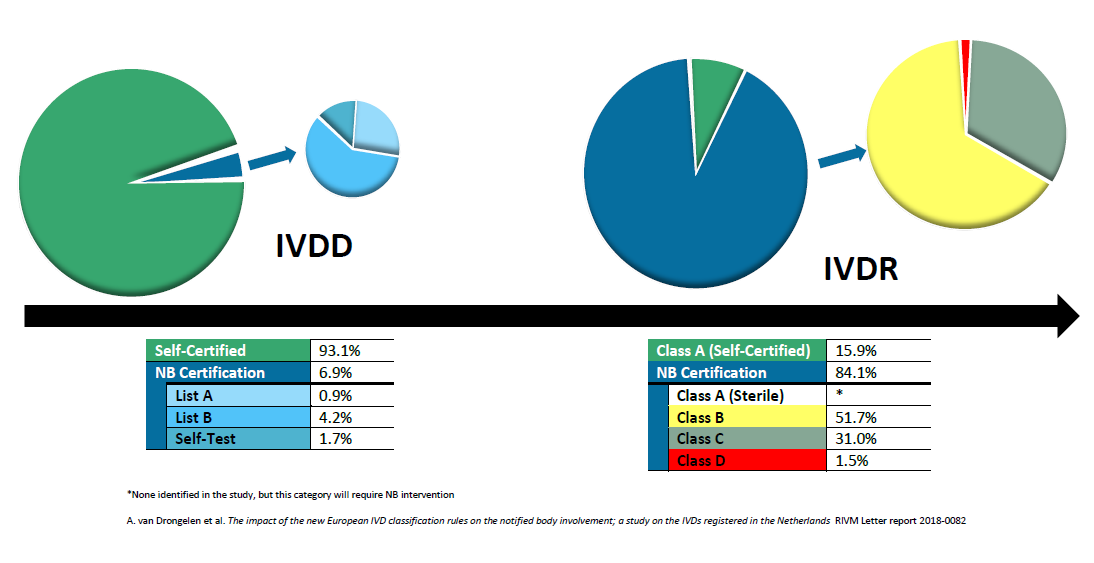
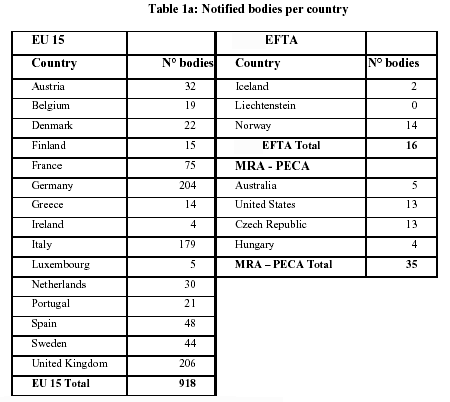
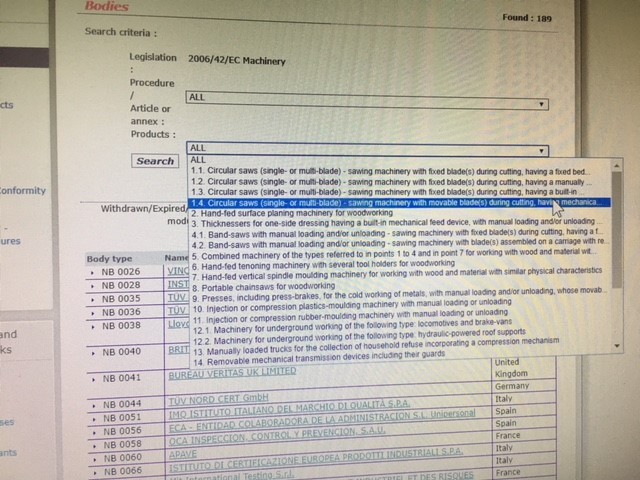
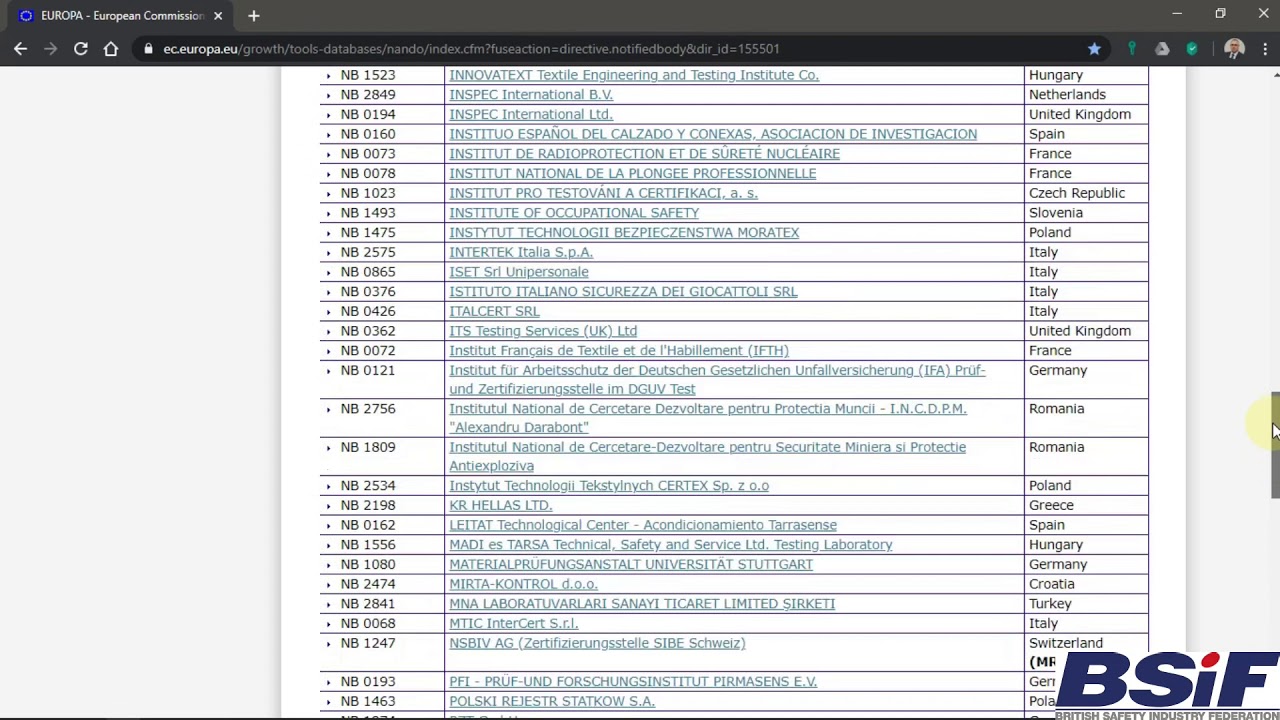
Organismos Notificados MDR (41): UDEM (Turquia) ON num. 2292 nuevo Organismo Notificado. Enhorabuena !!!

Auditing Organization (AO) versus Notified Body (NB) versus Registrar. What's the difference? – Oriel STAT A MATRIX – ELIQUENT Life Sciences Blog

complete list of notified bodies ,list of notified body,CE MARKING from NOTIFIED BODY ,how get ce certification for my product, CE marking CE mark CE certification CE approval CE certificate CE Logo
Availability and capacity of notified bodies to carry out conformity assessments for COVID-19 related medical devices and in vit



.jpg)