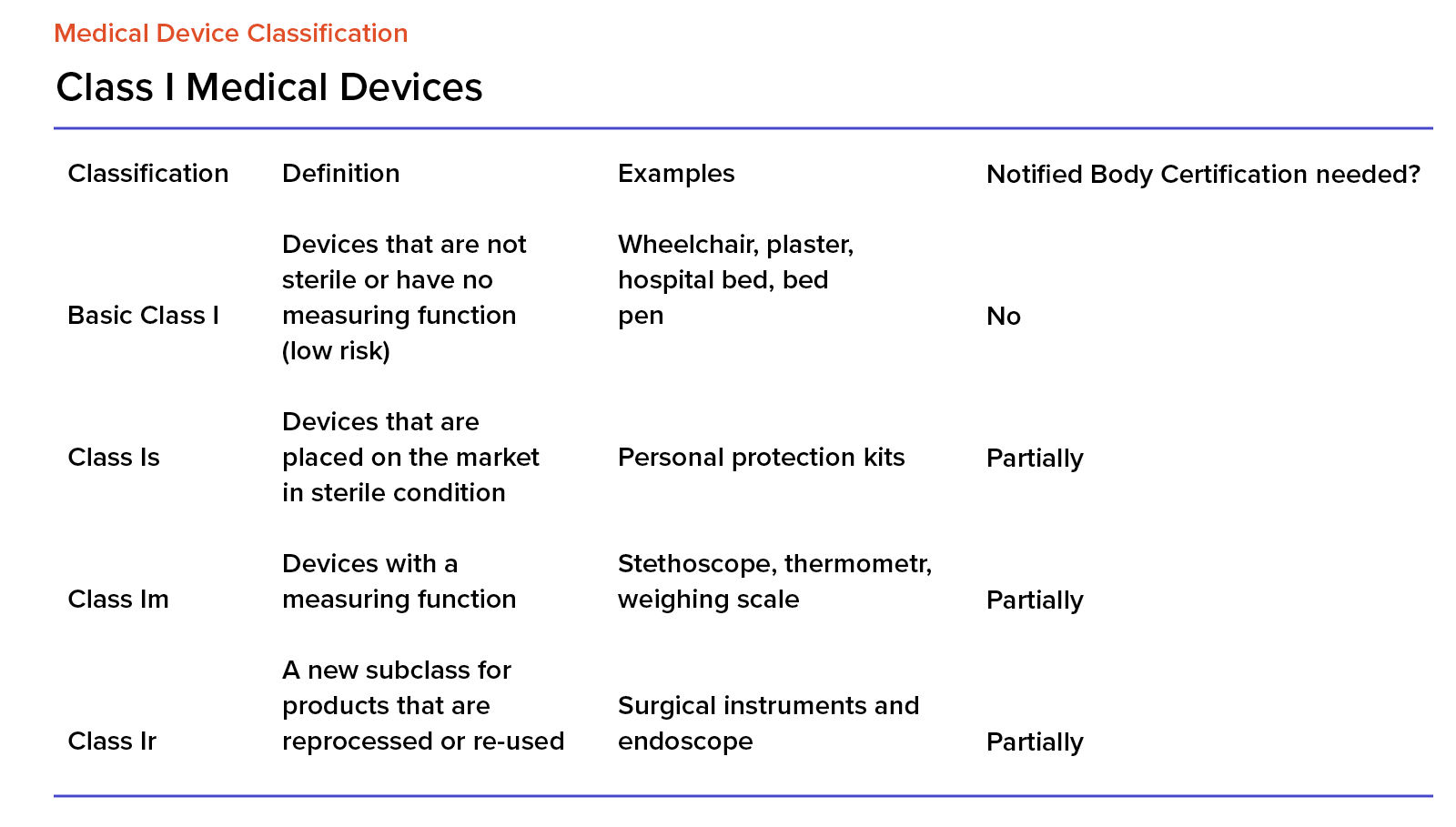
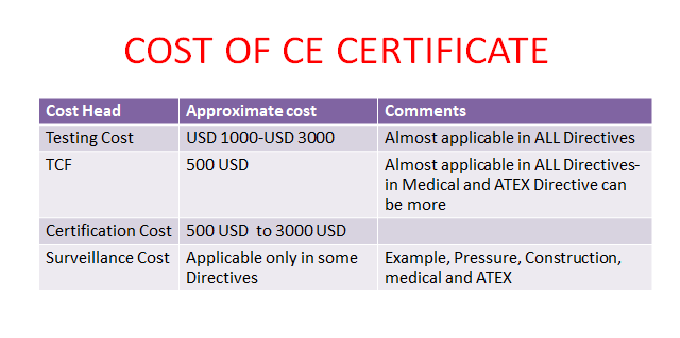
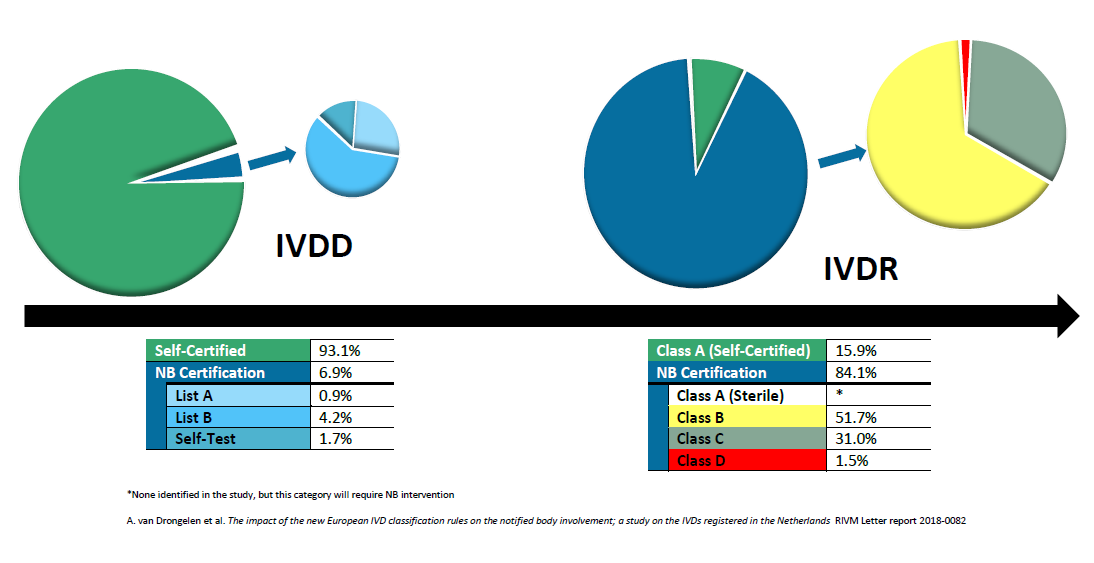
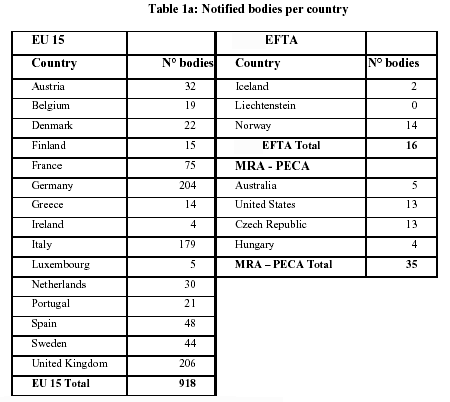
Organismos Notificados MDR (41): UDEM (Turquia) ON num. 2292 nuevo Organismo Notificado. Enhorabuena !!!

Check list of requirements for electrical equipment accordingto Low Voltage Directive 2014/35/EU, Annex I
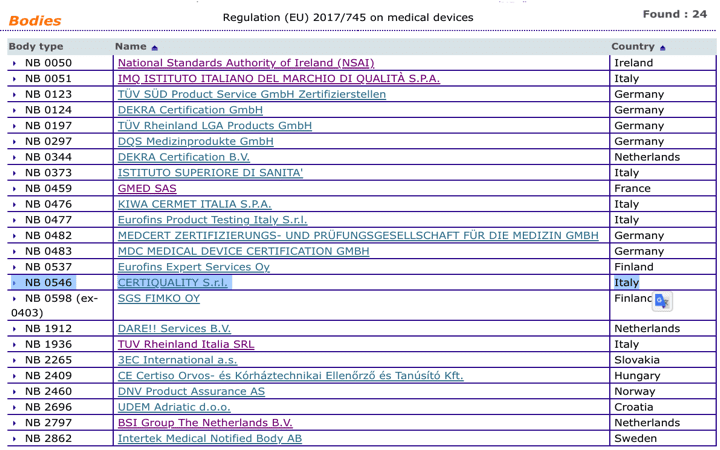
Organismos Notificados MDR (24): CERTIQUALITY (Italia) ON num. 0546 nuevo ON. Enhorabuena!!! | Red de Tecnologías Sanitarias y Productos Sanitarios

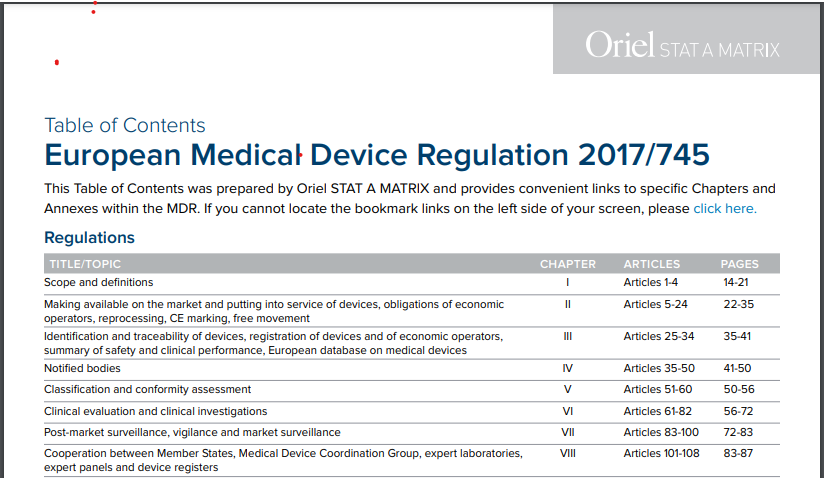
Which EU Notified Bodies Have Been “Designated” Under the MDR 2017/745 and IVDR 2017/746? – Oriel STAT A MATRIX – ELIQUENT Life Sciences Blog